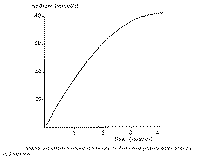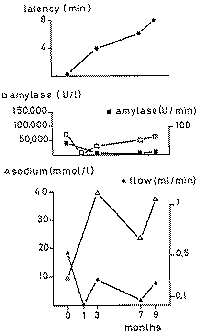|
Contents |
Sialometry
and sialochemistry |
|
|||||||||||||||||||||||||||||||||
|
|
K.
Graamans & H. P. van den Akker (eds). Diagnosis
of Salivary Gland Disorders,.139-161 1991 Kluwer
Academic Publishers. Printed in the Netherlands L.F.E.
MICHELS 1. Introduction The major salivary glands, particularly the parotid gland, are easily
accessible for saliva sampling techniques. The use of these techniques,
however, is limited by questions of interpretation and clinical value. Sialometry fails to provide information about
individual gland volume or about pathways and levels of innervation. Sialochemistry
appears to be very sensitive, but its specificity regarding the classical
pathology is low. There are no standard values for the main salivary
constituents. They should always be estimated in relation to flow rates and
water transport across the duct lining. Nevertheless, sialochemistry warrants
confidence in view of the results of experimental research and the
consistency of intra-individual measurements. Sialochemistry can be expected
to reveal the following: §
differentiation between normal and abnormal
function of the glands. §
information about gland dysfunction and its
impact on the oral environment, including the mucosa and periodontal
tissues. §
clues to homeostatic fluctuations as a result
of circulatory, innervatory, or hormonal adjustments. Secretion of saliva is an active and continuous process. Transport of
electrolytes is enhanced by sympathetic and parasympathetic stimulation (1).
Cholinergic activity is followed by water and electrolyte transport. After
B-sympathetic stimulation, protein synthesis and exocytosis become
predominant. A two-stage model for saliva production is widely accepted
(2-4). First stage: in the acinus, a chloride shift is
followed by sodium and water transport across the membranes and cell
junctions. Within the cell, cyclic AMP is the second messenger accompanying
protein synthesis and exocytosis following a- and 0-adrenergic agonists.
Cholinergic water transport requires calcium. Simultaneously a 20-fold rise
in capillary blood flow is observed. This 'primary secretion' is isotonic with plasma values. The main
proteins are a-amylase and the proline-rich proteins. The total protein
production remains, at about 1000 mg/l, far below plasma concentrations. In
addition to active water transport the contraction of myoepithelial cells is
an important flow-driving force. Second stage: the primary fluid is rendered hypo
tonic in the ductules by active sodium and bicarbonate reabsorption. Some
water is presumed to follow. In exchange, potassium and some proteins are
secreted. This process is assisted by membrane K-Na-ATPase. It is interesting
to note that reabsorption processes take time and reach steady-state values
at a given flow rate. Within normal functional limits, salivary flow rate and
sodium concentration show a good proportional relationship. At high flow
rates, the primary secretion will pass
through the pars striata too quickly to allow much reabsorption to occur, and
hence the sodium concentration will also be high. In 'resting saliva' the
sodium concentration falls to 1-2 mmol/l. Normally, the lining of the main
excretory ducts is highly impermeable to water and maintains a low osmolarity
of the ductal fluid. Particularly in cases of inflammation does equilibration of ductal
with interstitial fluids result in elevated sodium values in saliva. It is
obvious from the successive steps in secretion and reabsorption that
contradictory results can be obtained for sodium levels. This makes correct
interpretation rather difficult. They should therefore be interpreted with
some caution. Whole or mixed saliva is unsuitable for estimating glandular function.
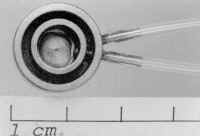
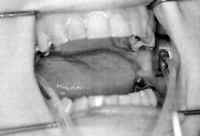
Therefore, the application of suction cups mounted simultaneously on both
parotid ducts is advisable (Figs. 1-3). Alternatively, a polyethylene
catheter (0.5-1.5 mm) can be introduced through the duct orifice (5). A
simple gustatory stimulus is provided by a 5 percent citric acid solution
applied to the tongue surface by means of a gauze pellet every 30 seconds.
Depending on the study design, the initially collected saliva (at 10 minutes)
should be discarded. Salivary studies are best done at fixed hours in order
to avoid circadian dissimilarities. The study of resting saliva is useful in
neurological disorders like athetosis with drooling and Parkinson's disease.
Measurements are always performed on both sides simultaneously. Small
differences may be observed due to unequal flow resistance as a result of
small variations in positioning of the collecting cups. In the correct
position, a catheter of 1 mm. diameter produces a counter pressure equal to 2
cm water. Since a counter pressure equal to a 10 cm water column results in a
20 percent increase in salivary output, chewing forces causing compression of
the duct should be avoided. Routine inspection of the parotid duct by gently pressing the gland is
of no value except in cases of gross ductectasis where a sudden spurt of
saliva will be seen. In contrast a sudden flow of saliva during massage is
normal in the submandibular gland. Absence of this phenomenon, for instance
in the presence of a calculus must be considered as a sign of abnormality.
4. Sialometry 4. 1. Flow rate Salivary flow rate is given as ml/min/gland. We consider the 'net'
flow to be the resultant salivary volume secreted by an activated section of
the gland minus the reabsorbed portion. Under 'resting' conditions the flow
rate of the parotid gland amounts to 0-0. 1 ml/min. After citric acid
stimulation the range is 0.5-1.5, ml/min. The functional reserve in top
secretors can reach up to ml/min. Stimulated values below 0.3 ml/min are
considered pathological. Elevated flow rates will be seen under conditions such
as gingivitis. recent prosthesis and dominant cholinergic activity in
Parkinson's disease, intoxication etc. Low values are found during the use
of, e.g., (tricyclic) antidepressants after duct disintegration caused by
inflammation or irradiation and after radical surgical treatment. The effects
are more dramatic in resting saliva on account of intensified water
reabsorption in the resting state. In some cases a functional reserve will
mask this effect.
4.2. Latency time If the initial fluid is not discarded, a latency time elapses between
the application of a stimulus and the appearance of saliva in the collecting
catheter. A period of about 20 seconds is found in normal glands. Both
flow rate and latency time are related to each other (Fig. 4). There is a
sharp rise of the latency time below, flow rates of 0.3 ml/min. Values
exceeding 60 seconds are therefore considered pathological. A long latency
time points to all kinds of diminished glandular function including the
existence of a very small gland. It is also a prominent feature of the dead
space in ductectasis and has been described as the 'fire-hose' phenomenon. A
large number of clinical observations in our own department indicate a close
relationship between long latency times and the sensation of xerostomia.
After irradiation, latent periods exceeding 10 minutes are seen. As is the case with other organs, it is difficult to estimate the
exact glandular volume. What matters, however, in clinical situations is the
functional capacity. This is illustrated by obvious changes in aging
glandular parenchyma. In spite of the loss of about 30 percent of secretory
tissue, there is little or no decrease in stimulated flow rate (6). 4.3. Salivary pressure By elevating the catheter system above a height of 10 cm. the salivary
flow slows and eventually ceases at 50-60 cm above the ductal orifice. By
interposing a water column of up to 10 cm within the collecting system the
flow rate increases unilaterally. As already pointed out, this initial
increase may well be compared with the effect of duct compression exerted by
masticatory muscles (5). Meanwhile a transepithelial reflux can be
demonstrated while duct permeability increases. As the salivary drive is
predominantly generated by myoepithelial cell contraction, it is of interest
to note a similarity in peaking values of salivary pressure during maximal
secretion as well as in cases of abnormal sodium retention, as shown in Fig.
5. In both groups. This may be attributed to the simultaneous participation
of an increasing number of acini with their corresponding myoepithelial
cells.
The choice of laboratory investigations should be based on presumed
relationships with intra glandular transport processes (sodium), intra
cellular synthesis (protein, amylase), and diffusion by plasma constituents
(urea). Saliva also influences the oral environment in a number of ways
(glycoproteins). Measurements are given as concentrations, c.q. mmol/1. This
facilitates the assessment of ion/water shift and osmotic values. Secreted
solutes, given as mmol/min or in mg/min (mmol/1 x ml/min), are useful in
judging acinar destruction, as in irradiation and aging. Routine laboratory
investigations include potassium calcium, sodium, chloride, bicarbonate,
urea, total protein, amylase, and osmolarity measurements.
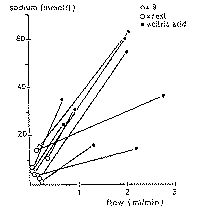
5.l. Sodium A number of membrane processes facilitate sodium and water transport
from the interstitial tissues into the acinar lumen. After both cholinergic
and sympathetic stimulation, plasma values are found in the primary fluid for
sodium, chloride, and bicarbonate. Potassium is slightly elevated. The
primary secretion is almost isotonic with plasma (2). Therefore the variable
sodium concentrations at different flow rates depend on changes during duct
passage. Within the ductules, the pars striata is responsible for sodium
chloride and bicarbonate reabsorption. As this transepithelial transport is
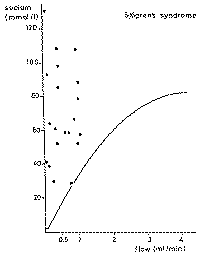
time consuming, values are flow-dependent (Figs. 6 and 7). Sodium
concentration is proportional to flow rates from 0. 1 to 2.5 ml/min. Here the formula, f(x) =
36(x) is applicable. During further duct passage, some water equilibration takes place and will
account for slightly elevated sodium concentrations at extremely low
flow rates. If, on the other hand, the parotid gland is stimulated maximally,
with flow rates over 2.5 ml/min., the sodium level tends to 'plateau'. This
stabilization of sodium concentrations at a fixed level is a challenging
phenomenon. It may be interpreted as an effect of enlarging the acinar
secreting surface by simultaneously activating more acini. In this situation,
there is no further increase in primary secretion per acinar unit. Therefore
the sodium gradient across the striated duct lining is stabilized. The sodium
concentration in the main duct tends to be unchanged as well. Meanwhile the
flow, i.e. the final salivary output, will be increased due to the
participation of a larger number of acini on account of a functional reserve
within the gland. Sodium plateau forming is observed in the smaller parotid
glands as well as in cases of diminished capillary blood flow. Thus, very low
plateau values, ranging from 1 to 2 mmol/l. are often seen in patients with
serious circulatory problems. Sodium re-absorption will be
accompanied by water reabsorption. There is, however, no information about
the amount of water reabsorption in relation to the initially formed fluid.
If increasing sodium reabsorption is accompanied by equivalent water
reabsorption, a steady state with zero saliva efflux during the night might
be postulated. When measuring salivary constituents, the effect of water reabsorption
on the concentration of the larger molecules must be taken into account. Some
increase of the larger molecules can be expected as a result of this water
shift. Damage of any kind to the striated duct will lead to decreased sodium
(and water) reabsorption. In contrast to this, leakage from the 'passive'
main duct results in water loss due to osmosis. We assume this continuous
osmotic drive to be responsible for the ecstasy of the main duct regularly
seen in chronic inflammation.
5.2. Bicarbonate Bicarbonate is part of an important buffer system in the oral cavity (2.
7). Reabsorption mainly takes place during passage through the intra lobular
duct. Usually, low concentrations of bicarbonate appear together with low
sodium. However, low values are also found coupled to small secretion rates,
even where sodium concentration is high, as in cases of inflammation or
irradiation. Except for extreme situations no correlation was found with the
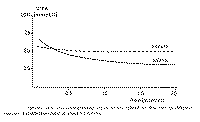
body acid-base balance. 5.3. Urea After bacterial breakdown the urea components provide another buffer
system in the oral cavity. A number of characteristics of urea make this
substance a useful tool in sialochemistry, notably its central production in
the liver, low molecular weight, and electric neutrality. These properties
allow a quantitative assessment of the reabsorption of water within the
gland. Acinar values of urea are slightly below plasma levels, probably
because of some flow-dependent molecular reflection (8). Laboratory- findings
will show a steady, state of urea concentration in saliva at 30 percent below
the plasma value, when salivary flow rates exceed 1 ml/min/gland. The mean
salivary urea approaches plasma urea values at flow rates of about 0.3
ml/min. It surpasses plasma values at flow rates beneath 0.3 ml/min (Fig. 8).
This isoconcentration point is modified by the sodium reabsorption activity.
This fairly, reliable flow-dependent urea gradient will gradually disappear
in situations of increased duct-wall permeability due to inflammation,
irradiation etcetera.
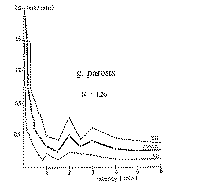
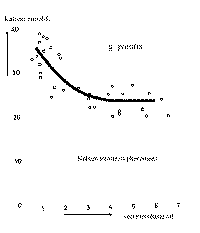
5.4. Potassium The salivary potassium concentration shows a more or less constant
level of 20-25 mmol/l. Incidental high values of up to 60 mmol/l are found, resembling
the results of 'stop flow' experiments. Elevated concentrations will be
observed in the first portion of saliva sampled after applying a stimulus
(Fig. 9). These 'rest transients' disappear in a collected volume of more
than 3 ml. Since active potassium secretion takes place mainly in the
striated duct. Low values (< 10 mmol/1) are seen after the destruction of
this ductal segment. The active potassium secretion may allow differentiation
between destruction by inflammation and irradiation. The striated duct
appears more radio-resistant than acinar tissue.
5.5. Protein The salivary glands play an active role in the synthesis of numerous
proteins. Abnormal proteins are also produced under exceptional conditions,
such as the development of tumours and nutritional deficiency. The exocrine secretion is dominated by a-amylase. Other constituents
are the (acid and base) proline-rich proteins, the immunoproteins, and the
growth factors. The two latter, as in the case of the glycoproteins, are
mainly produced in the ductules. Other substances, for example some blood
proteins and steroids, enter the duct via a transepithelial route. The mucins
(glycoproteins), which play an important role in oral functions, originate
mainly from the sublingual and the numerous smaller glands. Major health
problems will arise in inherited mucin disturbances. These are known to occur
in cases of cystic fibrosis. The condensed protein chains are stored in secretion granules with
special membrane characteristics. Undissolved inclusions will appear as
'spherulae' in saliva (2. 9. 10). These spherulae may be responsible for the
milky appearance seen at times in resting saliva and in cases of increased
sodium (and water) reabsorption. The specialized acinar functions of
producing, storing, and discharging secretory protein are mainly under the
influence of the sympathetic nervous system. Any such autonomic nervous system
disturbance will easily lead to derangement, frequently dominated by abnormal
storage and acinar swelling. The clinical effect is bilateral swelling of the
whole gland in the parotid and submandibular regions. Low a-amylase concentrations are seen in cases of starvation and after
acinar destruction and degeneration of the acinar cells. Elevated a-amylase
and total protein is to be expected in abnormal ductal water loss.
Furthermore, acute inflammation of the glands produces a rise in plasma and
urine amylase due to gross glandular leakage. This will be seen in mumps as
well as in the presence of a salivary calculus. The various functions of the salivary glands depend in every respect
on the body's main regulatory systems. The glands have high-energy demands
while they require flexible perfusion facilities. However, the body gives low
priority to the production of saliva compared to the maintenance of vital
organs such as the brain, kidney, or heart. In general, changes in capacity
or dynamics of the circulation are directly reflected in salivary gland
function by a diminished flow output and abnormal sodium retention. Therefore
blood investigations will be directed to a number of body- functions and
pathologic changes related to regulatory patterns. The important ones are the
blood count and the chemistry related to circulatory disorders, diabetes,
thyroid function, metabolic liver function, auto immune disorders, and the
consequences of the use of drugs. Repeated recording of the blood pressure
should not be neglected. 7. Diagnostic aids to salivary gland
disease Sialochernistry does not accommodate classical nosology. Different
diseases that have inflammatory processes in common will show the same
changes in their sialochemical patterns. These patterns are very sensitive.
At the same time, the specificity regarding classified diseases is low.
Therefore a correct diagnosis will always require a full clinical and
laboratory investigation. However. sialochemistry is a useful means of
chronologically, monitoring quantitative changes. 7.1. Inflammation Inflammation in the salivary glands is characterized by accumulation
of B-lymphocytes around the ducts and acinar cells, causing destruction
and/or proliferation (11). Atrophy of the acini may follow ductular
obstruction. Consequently, there is decreased sodium reabsorption and
potassium secretion in the striated duct, while water is drawn through the
disintegrating duct lining by osmosis. Initially, total protein and amylase
rise while the output in mg/min decreases. In chronic inflammation, the latency time may be prolonged while the
flow is elevated. In this situation, the latency is largely due to
ductectasis. It is assumed that a compensating mechanism that simultaneously
stimulates more acini is responsible for the elevated output. The continuous
osmotic drive across the duct lining is assumed to be the cause of the
ductectasis itself. The latter is a prominent feature of chronic
inflammation, as demonstrated by sialography. 7.2. Mumps Mumps, or epidemic parotitis is the most common of all salivary gland
diseases (5. 10). In about 50 percent of cases, clinical changes in the
glands are absent. Where swelling is prominent, oedema and a massive
accumulation of lymphocytes and plasma cells compress the salivary duct,
which may result in almost complete asialism. The diagnosis is made by
detection of a rise in antibody titer by complement fixation after two
estimations. Elevated values for serum and urine amylase are consistently
found. Differentiation of the isoamylases distinguishes between a rise due to
parotid as opposed to pancreatic pathology. In mumps the saliva shows a sharp
increase in the sodium concentration and an exceptionally low potassium
concentration, which approaches its plasma equivalents. Sodium values rise to
90-120 mmol/I while potassium values fall below 10 mmol/l. Aberrant values
may persist for months. 7.3. Recurrent
obstructive parotitis In children, repeated attacks of parotitis may affect one or both
sides. They generally follow a common cold and last a period of three to five
days. Histological, ductal damage by lymphatic invasion is observed (12).
This results in the extravasation of a contrast medium giving rise to the
'snowstorm appearance' of pseudectasis. As lymphatic tissue gradually
disappears by puberty, attacks will fade away in most patients. During the
acute phase, flow rates of saliva are reduced and areas of purulent necrotic
discharge are found from which physiologic oral flora can be cultivated.
Sialochemistry demonstrates all the signs of inflammation. During remissions,
the sialometric parameters will also recover. This behavior differs from that
seen in Sjögren's syndrome and after irradiation. At the moment, little is
known about the immunohistology of and the immunoproteins in saliva. Further
investigations should be conducted in this most interesting field. The flow
rate is often somewhat elevated in the latent periods of the disease,
indicating a small residual obstruction. 7.4. Sjögren's syndrome This clinical entity, that includes kerato-conjunctivitis sicca,
xerostomia and rheumatoid arthritis, probably depends on the presence of
activated T-lymphocytes and hyper reactive B-lymphocytes in exocrine organs
(10, 13). The auto immune behaviour of these lymphocytes expresses itself by
producing a large number of non-specific antibodies, which can be
demonstrated by laboratory investigation. The parenchyma of all the salivary
glands reveals destruction of the ductules, creating myoepithelial islands (14).
The acini are gradually lost. There is swelling of the major glands, at times
with redness and pain. In cases of purulent flow. Streptococcus viridans,
Klebsiella or Enterobacteriaccae can be cultured. While the 'primary' Sjögren
type is seen in all the salivary glands, a gland biopsy, from the lower lip
confirms the diagnosis if accumulation of IgM and IgG is demonstrated
(15-17). Sialochemistry. The multiple foci of gland
destruction and the degeneration of acinar cells is rapidly followed by
prolonged latency times and decreased flow. This is more pronounced in
resting secretion, while stimulated flow rates initially appear to be normal
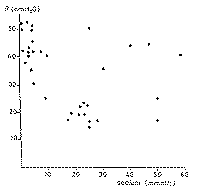
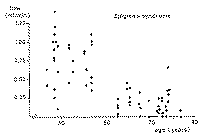
(Fig. 10). High sodium concentrations ranging from 60 to 100 mmol/1 are found
at any given flow rate (Fig. 11). The potassium concentration lies between 10
and 20mmol/1. The total protein production in mg/min is lower during the
disease while concentrations of protein persist at an elevated level. This
behavior parallels that of amylase.
Aberrant electrophoretic patterns may be seen. The typical inverse of the
saliva/scrum urea graph at about 0.3 ml/min flow rate is lost. This indicates
leakage of both water and urea and might be responsible for the extremely low
secretion rates at rest. This is also in accordance with ductectasis. The
lymphocyte infiltration may be the cause of the ductal damage and
pseudectasis seen in sialography. If a malignant lymphoma develops
incidentally, abnormally high calcium concentrations are found even
surpassing plasma values. Further laboratory investigations, including electrophoresis,
immunochemistry, and blood counts, should always be performed. While
erythrocyte sedimentation rates above 60 mm and globulins > 18 g11 alone
are not pathognomonic, their appearance in combination with the described
sialochemistry is highly suggestive of Sjögren's syndrome. Some studies emphasize the sharp rise in glandular kallikrein and its
significance in maintaining inflammation. Improvement is seen after
intravenous administration of its antagonist aprotinin (Trasylol) (18) with
kallikrein concentrations in saliva normalize within 24 hours. 7.5. Sarcoidosis (Heerfordt's disease) As a part of systemic sarcoidosis. granulomatous foci may be seen in
the salivary glands, which may even cause bilateral swelling of the parotid
gland. This epithelioid sialadenitis does not lead to serious functional
glandular disorders. The flow is elevated as a result of slight tissue
compression. Sialochemistry fails to reveal inflammatory changes. Kallikrein
is reported to be low (5). While angiotensin converting enzvme (ACE) will be
elevated in the plasma, it is not known whether or not ACE is present in the
saliva. Normally, this enzyme is absent in saliva. 8. Irradiation Radiotherapy of malignancies of the head and neck causes rapid and
severe destruction of the parenchyma of the glands. A loss of function of up
to 50 percent is measured within the first week ( 10). In the most
susceptible serous glands, cell enlargement, formation of vacuoles with
degranulation, and finally necrosis develop. Capillary walls are thickened
and atrophy of the nerves follows after several months. Sialochemistry
immediately reveals inflammatory changes (19). An increased latency time of
10 minutes or more is not exceptional. Flow falls to zero while the rise in
sodium level is steep (80-120 mmol/1). Potassium values are stable, while
amylase diminishes both in concentration and production (Fig. 12). These
alterations indicate decisive acinar destruction and relative radio
resistance in the ductal segments. Some repair is seen in most cases.
However, there will never be a return to pre-therapy values. While the
effects of radiotherapy are generally described as quantitative, the
subjective sensations and experiences are not. Complaints of intense oral
dryness and sticky saliva are the rule. A shift in oral flora towards the
gram-negatives will also be responsible for superficial mucositis and
distorted oral perception (20).
9. Sodium retention dysfunction
syndrome The normal proportional relationship between salivary flow and sodium
concentration is absent in a number of persons. Not only are smaller flow
rates measured but also the sodium concentration is relatively low and
fluctuates around a steady state of 2.5 mmol/1 (Fig. 13) while most other
substances are slightly raised. In some cases, the saliva has a milky
appearance. These indications of sodium retention dysfunction syndrome are
fairly typical and may be found at all ages, although they are more prevalent
in later life. The entity is seldom described in the literature (21).
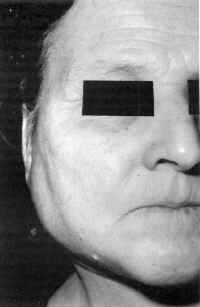
Prominent clinical signs are the sensation of a dry mouth and incidental
unilateral painless swelling of the parotid gland for a few hours e.g. during
breakfast (Fig. 14). With some exceptions, the dysfunction persists through
life. Impaired gland perfusion is frequently found. This may be caused by
arterial wall thickening or by 'homeostatic' mechanisms of the circulatory
system in favour of other important organs. Risk factors can be listed in
subgroups such as both hyper- and hypo tension. cardiac failure, local and
systemic oedema from other causes, and dehydration. A sudden onset of sodium
retention is seen after arteriovenous shunt in hemodialysis. This phenomenon
is not related to the time at which dialysis was actually performed (22. 23).
Increased sodium reabsorption may be due to hormonal effects on the
sodium/potassium exchange rate or even to elongation of the striated duct (22).
Both mechanisms should be regarded as minor factors in man. One hypothesis is diminished perfusion of the gland followed by
intensified release of neural impulses. This simultaneously activates more
acini, thus enlarging the secreting surface. The adaptation partially
restores the total fluid output, while the flow per ductule is not
substantially changed. In this way, sodium reabsorption remains in a steady
state despite the increasing flow rate. The functional shift described has
its analogy in the plateau sodium values reached in the maximal stimulated
normal parotid gland. In this situation, maximal output is also finally
reached by simultaneously activating more acini. Further support for this
hypothesis concerning sodium retention dysfunction is given by salivary
pressure measurements. As can be seen in the graph of Fig. 5, the upper
pressure levels are recorded at the high sodium concentrations as well as at
the low values. They represent both the top secretors and the sodium
retention dysfunction group, suggesting a similar increase in the number of
participating acinar rnyoepithelial units. Under normal conditions, salivary
glands never function as a whole. Their sequential lobular activity is
clearly visualized during surgical procedures using a nerve stimulator. It is
important to note that sodium retention dysfunction syndrome is seen not only
in the parotid but also in the submandibular glands. Under these conditions
the deregulated glands are 'at risk' of superimposed pathology. Whereas unilateral painless swelling for short periods is the usual
symptom, in some patients the reverse is encountered, These patients complain
of bilateral swelling with painful tension and only incidental hours or days
of regression and a normally shaped face. It is suggested that they have very
large parotid glands or else an intensified sodium reabsorption rate in the
striated duct. Swelling may be due to accompanying water reabsorption. Particular
in this group, a transitory swelling is seen after the use of lipiodol in
sialography. Regression of the swelling is achieved by reducing the
reabsorption time by administration of pilocarpine. Sometimes amelioration is
seen during the use of spironolactone. The sialochemistry, of the latter
group is characterized by increased or even normal flow rates and an
equilibration point of serum/saliva urea at flow rates >0.3 ml/min. This
shift strongly suggests increased water reabsorption.
The extended latency time, the reduced flow with sodium retention, and
low bicarbonate values accompanying
the sodium retention dysfunction syndrome may have a profound impact on the
oral environment. This kind of gland dysfunction is in fact a common finding
in periodontal disease, superficial glossitis, glossodynia, and taste
disorders. Evaluation of salivary gland function therefore is indicated in a
broad range of clinical signs and symptoms. 10. Sialadenosis The term sialadenosis is applied to non-inflammatory- disorders of the
parenchyma of the salivary glands. These disorders are rooted in metabolic
and secretory unbalance and are frequently, accompanied by painless bilateral
swelling of the major glands, especially of the parotid gland (25. 26). The
swelling persists for years. The condition is seen in nutritional and
metabolic defects (anorexia nervosa. alcohol abuse), endocrine disorders
(diabetes), neurogenic disorders and unbalance after prolonged use of
B-sympathomimetic drugs. Histology shows excretory disturbances with light or
dark staining and swelling of the acinar cells. Hypertrophy or hyperplasia
may also be found. A primary neuropathy is suggested (27). No characteristic pattern can be identified in sialochemistry. The swelling
itself may cause a slightly elevated flow rate due to pressure effects. The
failure of the exhausted acinar cells is reflected by a low
amylase concentration and output parallel to the total protein curves.
However, both inhibition and stimulation of acinar proteins seems possible. 11. Salivary gland disease in terminal
illness A painful unilateral swelling of the parotid gland is occasionally,
reported in seriously ill patients. It is sometimes referred to as nosocomial
parotitis because of its prevalence during hospitalisation. When purulent
discharge appears from the duct orifice, the cultivated flora frequently,
originate from the digestive tract. Circulatory failure and uremia with
consequent xerostomia often dominate the clinical picture. If sialochemistry,
is performed it will reveal a markedly reduced secretory flow with distinctly
low sodium concentrations in the non-affected gland and very high
concentrations on the diseased side. As in other ascending infections of the
glands, the healthy gland provides insight into the pathogenesis of this
condition. 12. Tumours of the salivary glands Until now, the contribution of sialometry and sialochemistry to the
diagnosis and differentiation of salivary, gland tumours has been small.
Early indications of any- disorder in gland metabolism or protein synthesis
are neither to be found nor to be expected because of the isolated and
nodular nature of most tumours. However abnormal function of secreting tissue
may contribute to the production of tumour markers which will
direct attention to special cells or sites in the
secretory system (28). In this field immunohistology leads the way. In the
future, cytodiagnosis of cells in salivary samples should be strongly
encouraged. This will also prove
helpful in differentiating between a neoplasm and inflammation. A striking
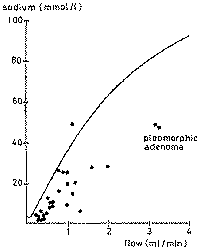
feature, not easy to explain is the relatively extreme bilateral sodium
retention in parotid saliva within the pleomorphic adenoma group (Fig. 15).
13. The effects of drugs A detailed description of the numerous drugs that influence glandular
function is beyond the scope of this section. However, some general remarks
should be made. Most anticholinergics, including the majority of antihistaminics,
tricyclic antidepressants, and anti -Parkinson's drugs, suppress the pulse
frequency of the salivatory nucleus. Apart from age-dependent changes during
prolonged drug administration, these effects are reversible. In
sialochemistry, the stimulated saliva values in this group come very close to
those of the resting saliva. There is a small risk of obstructive oedematous
swelling of the parotid gland on waking up, probably due to adhesion of the
duct orifice lining. Accelerated flows are seen after administration of
cholinergics, with sodium and bicarbonate concentrations corresponding to the
flow rates. The output and concentration of total protein amylase and calcium
are increased while potassium is diminished. Apart from ample experimental research in sialadenosis, the clinical
effects of sympathicomimetics have not been fully evaluated. Beta-mimetics
facilitate the expulsion of salivary proteins by increased myoepithelial
contraction and also enlarge a pre-existing ectasia. In sodium retention
dysfunction syndrome, no immediate improvement is seen after drug-induced
dilatation of the peripheral vessels. Nevertheless, normalized function is
sometimes observed after administration of diuretics. The immunosuppressive myelo-suppressive and cytotoxic effects of
chemotherapeutic agents profoundly influence salivary gland function (29).
Reduced flow and inflammatory changes dominate in sialochemistry. Obstructive
and painful swelling is infrequent but predicts a terminal course in these
cases. 14. Conclusion Sialometry and sialochemistry should be regarded as valuable
diagnostic tools that may well prove useful in comparing gland function on
the two sides. They are also reliable in intra-individual follow-up studies.
Despite the lack of standard mean values and the absence of interdependent
parameters (30) it is possible to make a dependable assessment of gland
function related to the systemic background and of the risk factors in oral
balance. The main drawback of these techniques is their lower
specificity for the classical entities in clinical pathology.
Unfortunately, communication and cooperation between research groups and
clinical workers is inadequate. Cooperation must be promoted. Also
consensus-building should be fostered with regard to a protocol for
investigations.
1. Garret J. Innervation of salivary glands. Neurohistological and functional
aspects. In: Sreebny
L.M. ed. The salivary system. Boca Raton: CRC Press. 1987: 69-93. 2.
Young JA. Cook DE, Lennep FW van, Roberts M, Secretion by the major salivary
glands. In: Johnson
R. ed. Physiology of the gastrointestinal tract. New York: Raven Press. 1987:
773-815
3. Izutsu KT. Salivary electrolytes and fluid production in health and
disease. In: Sreebny LM, ed. The
salivary system. Boca Raton: CRC Press, 1987: 95-116.
4. Baurn BJ. Regulation of salivary system. In: Sreebny LM ed. The salivary systern.
Boca Raton:
C.R.C. Press, 1987: 123-131. 5. Mason
DK, Chisholm DM. Salivary glands in health and disease. London: Saunders,
1987: 249. 6. Scott J.
Structural age changes in salivary glands, In: Ferguson DB, ed. The aging
mouth. Basel: Karger. 1987:
40-61.
7. Nieuw Amerongen A van. Speeksel en speekselklieren. Alphen
a/d Rijn: Samsom Stafleu,1988 8. Leaf A.
Transport of urea across a living membrane. In: Schmidt-Nielsen B. ed. Urea and
the Kidney. Amsterdam: Exe Med Found, 1970: 83-88. 9. Tandler
B. Riva A. Salivary glands. In: Mjör IA, Feyerskov O, eds. Human oral
embryology and histology. Copenhagen: Munksgaard, 1986: 243-284. 10. Harrison
JD et al. Ultra structural morphology of secretory granules of
submandibular and parotid salivary glands of man. Arch Oral Biol 1987: 32:
229.
11. Seifert J et
al. Speicheldrüsenkrankheiten. Stuttgart: Thieme. 1984. 12. Patey
DH. Thackray AG. Chronic sialectic parotitis in the light of pathology
studies in parotid material. Br J Surg 1955; 43: 43-50,
13. Talal L. Overview of Sjögren's syndrome. J Dent Res . 1987; 66:
672-674. 14. Saku T,
Okabe H, Immunohistochernical and ultra structural demonstration of keratin
in epi-myoepithelial islands of auto immune sialadenitis in man. Arch Oral
Biol 1984; 29: 687 689. 15. Scully
C, Sjogren's syndrome. Clinical and laboratory features, immunopathogenesis
and management. Oral Surg Oral Med Oral Pathol 1986; 62: 510-523. 16.
Stuchell RN, Mandel ID, Baurmash H. Clinical utilization of sialochemistry in
Sjögren's syndrome. J Oral Pathol 1984; 13: 303-309. 17. Hené RJ, Wilde PCM de, Kater L. De betekenis van
cle sublabiale speekselklierbiopsie als diagnose bij het syndroom van
Sjögren, de ziekte van Besnier Boeck en amyloidosis. Ned Tijdschr Geneeskd
1982; 126: 1027-1033. 18. Deeg M. Maier H, Adler D. Zur Therapie der
chronisch- rezidivierenden Parotitis. In: Weidauer H. Maier H. eds.
Speicheldrüsenerkrankungen. Berlin: Springer, 1988: 29-35. 19. Marks JE et al. The effects
of radiation on parotid salivary function. Int J Radiat Biol 1981; 7:
1013-1019.
20. Spijkervet FKL, Irradiation mucositis and oral flora. Groningen: thesis. 1989, 21. Rauch S. Natriumretinierende Sialose. Arch Klin
Exp Ohren Nasen Kehlkopfheilkd 1967: 188: 525-528. 22. Lichte JR, Mulder AW, Michels LFE. Parotid
gland dysfunction in haemodialysis patients. Neth J Med 1983~ 26: 39-43. 23. Shannon
I, Feller RP, Eknoyan G, Suddick RP. Human parotid saliva urea in renal
failure and during dialysis. Arch Oral Biol 1977; 22: 83-86. 24.
Junqueira LCU. Control of cell secretion. In: Schneyer LH, Charl A, eds.
Secretory mechanisms of salivary glands. New York: Academic Press. 1967: 286-302.
25. Rauch S. Die Speicheldrüsen des Menschen. Stuttgart: Thieme.
1959. 26. Chilla
R, Sialadenosis of the salivary glands of the head. In: Pfaltz CR. ed. Sialadenosis and sialadenitis.
Basel: Karger. 1981: 1-38.
27. Donath K. Die Sialadenose der Parotis. Stuttgart: Fischer. 1976. 28. Otto HF et al. Immunohistologische
Charakterisierung maligner Speicheldrüsentumoren. In: Weidauer H, Maier H,
eds. Speicheldrusentumoren. Berlin: Springer, 1988: 53-67. 29. Peterson D. Sonis S, eds. Oral
complications of cancer chemotherapy, The Hague: Nijhoff, 1983, 30. Blomfield J et al. Interrelationships
between flow rate. amylase, calcium, sodium, potassium and inorganic
phosphate in stimulated human parotid saliva. Arch Oral Biol 1976; 21:
645650. |
figure 1
figure 2
figure 3
figure 4
figure 5
figure 6
figure 7
figure 8
figure 9
figure 10
figure 11
figure 12
figure 14
figure 15
|
|||||||||||||||||||||||||||||||||
|
|
|